Protactinium
91
Pa
Groep
n.v.t.
Periode
7
Blok
f
Protonen
Elektronen
Neutronen
91
91
140
Algemene Eigenschappen
Atoomnummer
91
Atomair gewicht
231,03588
Massa Getal
231
Categorie
Actiniden
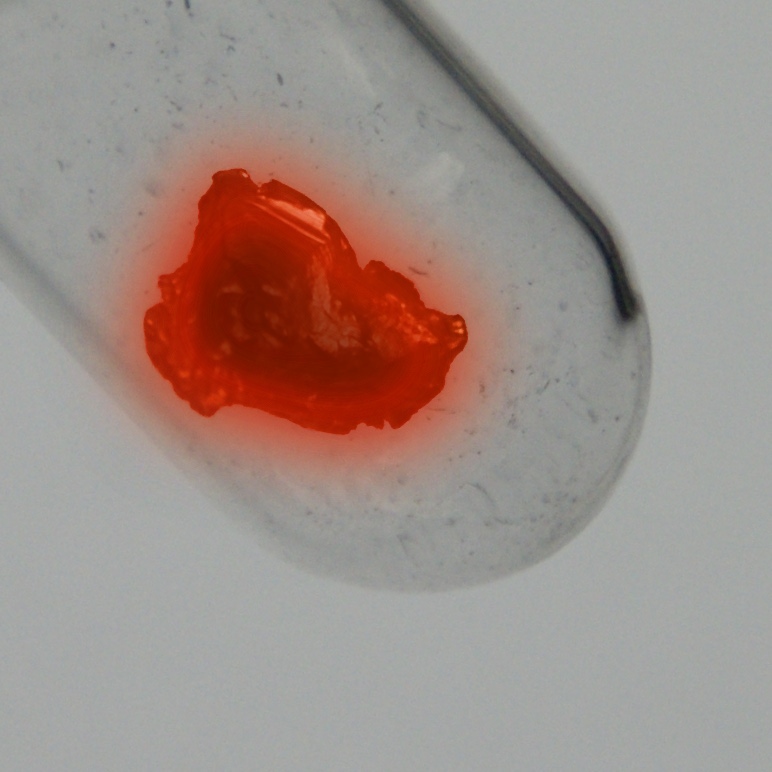
Kleur
Zilver
Radioactief
Ja
Van het Griekse protos betekenend eerste
Kristalstructuur
Gecentreerd Tetragonaal
Historie
In 1900 isoleerde William Crookes protactinium als een intens radioactief materiaal uit uranium.
Protactinium werd voor het eerst geïdentificeerd in 1913 door Kasimir Fajans en Oswald Helmuth Göhring in Duitsland.
Een stabieler isotoop van protactinium werd in 1917 ontdekt door Otto Hahn en Lise Meitner aan het Kaiser Wilhelm Instituut in Berlijn.
Protactinium werd voor het eerst geïdentificeerd in 1913 door Kasimir Fajans en Oswald Helmuth Göhring in Duitsland.
Een stabieler isotoop van protactinium werd in 1917 ontdekt door Otto Hahn en Lise Meitner aan het Kaiser Wilhelm Instituut in Berlijn.
Eletronen per schil
2, 8, 18, 32, 20, 9, 2
Electronconfiguratie
[Rn] 5f2 6d1 7s2
Protactinium is een van de zeldzaamste en duurste natuurlijk voorkomende elementen
Fysieke Eigenschappen
Fase
Vast
Dichtheid
15,37 g/cm3
Smeltpunt
1841,15 K | 1568 °C | 2854,4 °F
Kookpunt
4300,15 K | 4027 °C | 7280,6 °F
Fusiewarmte
15 kJ/mol
Verdampingswarmte
470 kJ/mol
Specifieke Warmtecapaciteit
- J/g·K
Overvloedig aanwezig in de aardkorst
9,9×10-13%
Overvloedig aanwezig in het universum
n.v.t.
CAS-nummer
7440-13-3
PubChem CID nummer
n.v.t.
Atoomeigenschappen
Atoomstraal
163 pm
Covalentiestraal
200 pm
Electronegativiteit
1,5 (Pauling schaal)
Ionisatiepotentiaal
5,89 eV
Atoomvolume
15,0 cm3/mol
Thermische geleiding
0,47 W/cm·K
Oxidatietoestanden
3, 4, 5
Toepassingen
Vanwege zijn schaarste, hoge radioactiviteit en hoge giftigheid zijn er momenteel geen toepassingen voor protactinium buiten wetenschappelijk onderzoek.
Met de komst van zeer gevoelige massaspectrometers is een toepassing van 231Pa als tracer in geologie en paleoceanografie mogelijk geworden.
Protactinium-231 gecombineerd met thorium-230 kan worden gebruikt om mariene sedimenten te dateren.
Met de komst van zeer gevoelige massaspectrometers is een toepassing van 231Pa als tracer in geologie en paleoceanografie mogelijk geworden.
Protactinium-231 gecombineerd met thorium-230 kan worden gebruikt om mariene sedimenten te dateren.
Protactinium is giftig en zeer radioactief
Isotopen
Stabiele isotopen
-Instabiele isotopen
212Pa, 213Pa, 214Pa, 215Pa, 216Pa, 217Pa, 218Pa, 219Pa, 220Pa, 221Pa, 222Pa, 223Pa, 224Pa, 225Pa, 226Pa, 227Pa, 228Pa, 229Pa, 230Pa, 231Pa, 232Pa, 233Pa, 234Pa, 235Pa, 236Pa, 237Pa, 238Pa, 239Pa, 240Pa