Radium
88
Ra
Groep
2
Periode
7
Blok
s
Protonen
Elektronen
Neutronen
88
88
138
Algemene Eigenschappen
Atoomnummer
88
Atomair gewicht
[226]
Massa Getal
226
Categorie
Aardalkalimetalen
Kleur
Zilver
Radioactief
Ja
Van het Latijnse woord radius betekenend straal
Kristalstructuur
Ruimtelijk-gecentreerde Kubus
Historie
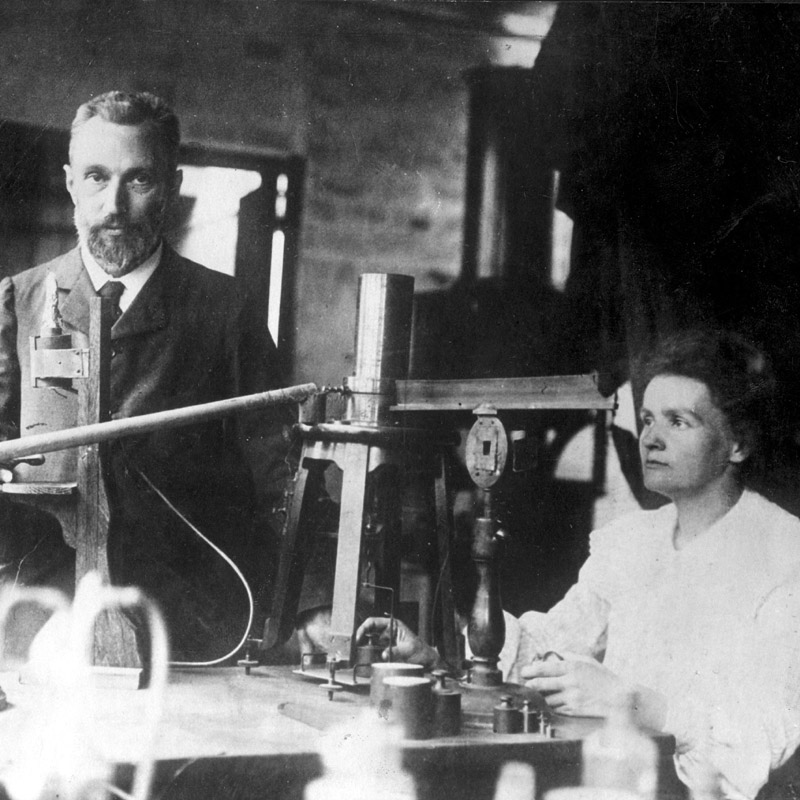
Radium werd ontdekt door Marie Curie en Pierre Curie in 1898.
Ze extraheerden de radiumverbinding uit een uraninietmonster.
Radium werd in 1910 door Marie Curie en André-Louis Debierne in metallische toestand geïsoleerd door elektrolyse van radiumchloride met behulp van een kwikkathode en destillatie in een waterstofgasatmosfeer.
Ze extraheerden de radiumverbinding uit een uraninietmonster.
Radium werd in 1910 door Marie Curie en André-Louis Debierne in metallische toestand geïsoleerd door elektrolyse van radiumchloride met behulp van een kwikkathode en destillatie in een waterstofgasatmosfeer.
Eletronen per schil
2, 8, 18, 32, 18, 8, 2
Electronconfiguratie
[Rn] 7s2
Radium geeft een karmijnrode kleur aan een vlam
Fysieke Eigenschappen
Fase
Vast
Dichtheid
5,5 g/cm3
Smeltpunt
973,15 K | 700 °C | 1292 °F
Kookpunt
2010,15 K | 1737 °C | 3158,6 °F
Fusiewarmte
8 kJ/mol
Verdampingswarmte
125 kJ/mol
Specifieke Warmtecapaciteit
- J/g·K
Overvloedig aanwezig in de aardkorst
9,9×10-12%
Overvloedig aanwezig in het universum
n.v.t.
CAS-nummer
7440-14-4
PubChem CID nummer
6328144
Atoomeigenschappen
Atoomstraal
-
Covalentiestraal
221 pm
Electronegativiteit
0,9 (Pauling schaal)
Ionisatiepotentiaal
5,2784 eV
Atoomvolume
45,20 cm3/mol
Thermische geleiding
0,186 W/cm·K
Oxidatietoestanden
2
Toepassingen
Radium werd vroeger gebruikt in zelflichtgevende verf voor horloges, nucleaire panelen, vliegtuigschakelaars, klokken en instrumentenpanelen.
Radiumchloride werd gebruikt in de geneeskunde om radongas te produceren dat op zijn beurt werd gebruikt als kankerbehandeling.
Het isotoop 223Ra wordt momenteel onderzocht voor gebruik in de geneeskunde als kankerbehandeling van botmetastasen.
Radiumchloride werd gebruikt in de geneeskunde om radongas te produceren dat op zijn beurt werd gebruikt als kankerbehandeling.
Het isotoop 223Ra wordt momenteel onderzocht voor gebruik in de geneeskunde als kankerbehandeling van botmetastasen.
Radium is zeer radioactief en kankerverwekkend
Isotopen
Stabiele isotopen
-Instabiele isotopen
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra